Introduction: CAR T-cell therapy is currently being evaluated in non-oncologic autoimmune diseases (Mougiakakos D et al. N Engl J Med 2021; Müller F et al. Lancet 2023). In a cohort of patients (pts) with SLE, CD19 CAR T-cell therapy resulted in B-cell depletion and treatment-free, durable remission per Definitions of Remission In SLE (DORIS), suggesting that CD19 CAR T therapy may be feasible, well-tolerated, and effective for patients with SLE (Mackensen A et al. Nat Med 2022). Baseline traits associated with greater manufacturing success and lower toxicity profiles for CAR T have been identified in hematologic oncology (Mashadi-Hossein A et al. J Clin Oncol 2023; Rytlewski J et al. J Clin Oncol 2022) including age, bone marrow function, inflammatory state, and tumor burden. Here, we compared baseline traits in pts with active SLE versus R/R LBCL to predict the potential manufacturing success and toxicity profile for the ongoing phase 1 study to evaluate the investigational NEX-T TM CAR T-cell product BMS-986353 (CC-97540) in pts with severe, refractory SLE (NCT05869955).
Methods: This post hoc analysis compared 42 quantitative baseline traits between pts with active SLE from PAISLEY (NCT03252587; Morand E et al. Arthritis Rheumatol 2023) and pts with R/R LBCL from TRANSCEND (NCT02631044; Abramson J et al. Lancet 2020). Baseline traits associated with improved manufacturing success and/or lower toxicity profile for CAR T in hematologic oncology were compared (Mashadi-Hossein A et al. J Clin Oncol 2023; Rytlewski J et al. J Clin Oncol 2022). A Wilcoxon rank-sum test was used to compare pt cohorts. Data are median (95% confidence interval).
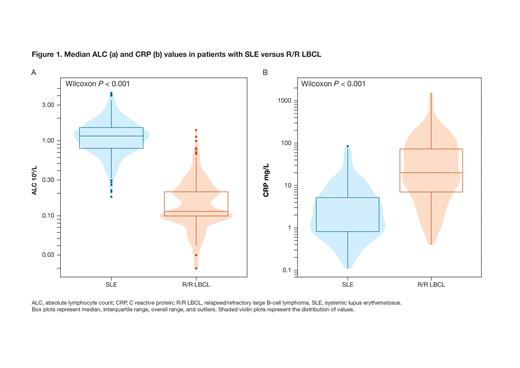
Results: This analysis included 363 pts with SLE and 256 pts with R/R LBCL. Of 42 baseline traits compared, 36 had nominal P values ≤ 0.05; 33 of those were ≤ 0.001. High baseline absolute lymphocyte count (ALC), platelets (PLT), and hemoglobin (Hgb) were previously associated with both improved manufacturing success and a lower toxicity profile for CAR T; pts with SLE had higher ALC, PLT, and Hgb vs R/R LBCL (ALC: 1.15 10 9/L [1.07-1.20 10 9/L] vs 0.10 10 9/L [0.10-0.13 10 9/L], P < 0.001, Figure 1A; PLT: 239 10 9/L [232-249 10 9/L] vs 144 10 9/L [129-154 10 9/L], P < 0.001; Hgb: 128 g/L [126-130 g/L] vs 98.0 g/L [95.0-100.0 g/L], P < 0.001), which is predictive of both improved manufacturing success and potentially lower toxicity. Young baseline age and high red blood cell (RBC) counts were previously associated with improved manufacturing success, and pts with SLE were younger and had higher RBC counts vs R/R LBCL (age: 40 years [38-41 years] vs 63 years [61-64 years], P < 0.001; RBCs: 4.30 10 12/L [4.20-4.40 10 12/L] vs 3.13 10 12/L [3.05-3.20 10 12/L], P < 0.001), predicting improved CAR T manufacturing success. Baseline traits reflecting tumor burden and/or bone marrow involvement that have been previously associated with higher toxicity, such as high C reactive protein (CRP; Figure 1B), high lactate dehydrogenase (LDH), and low hematocrit (Hct), were different between pts with SLE vs R/R LBCL (CRP: 1.92 mg/L [1.60-2.48 mg/L] vs 20.0 mg/L [15.5-31.8 mg/L], P < 0.001; LDH: 184 U/L [179-191 U/L] vs 282 U/L [251-317 U/L], P < 0.001; Hct: 39.0% [39.0-40.0%] vs 29.6% [28.7-30.5%], P < 0.001), and predict potentially lower toxicity.
Conclusions: Comparison of baseline pt data from trials in SLE vs R/R LBCL suggests that pts with SLE have traits that predict improved manufacturing success and lower rates/severity of common CAR T-associated toxicities such as cytokine release syndrome (CRS) and neurological events. A key difference between cohorts is the potential for high tumor burden in R/R LBCL which has been previously associated with CAR T toxicity. Our results show marked differences between cohorts for tumor burden surrogate measures (CRP, LDH, Hct), bone marrow function, inflammatory state, and age which we hypothesize may potentially lead to improved CAR T manufacturing success and lower risk of CRS and/or neurological events for patients with autoimmune disorders vs R/R LBCL. Further research is needed to understand if CAR T-cell therapy may provide a promising treatment option for pts with SLE and other autoimmune conditions. The ongoing phase 1, multicenter, open-label study will evaluate the safety, feasibility, and preliminary efficacy of BMS-986353 (CC-97540) in patients with severe, refractory SLE.
Study support: Bristol Myers Squibb
Disclosures
Mueller:Miltenyi, BMS, Novartis, Gilead, Janssen, Incyte, AstraZeneca, Abbvie, Sobi, Beigene: Honoraria; AstraZeneca, BMS, Gilead, Janssen, Miltenyi biomedicine, Novartis: Consultancy; BMS, AstraZeneca, Gilead: Research Funding. Rytlewski:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Adaptive Biotechnologies: Current equity holder in publicly-traded company. Wu:Bristol Myers Squibb: Current Employment. Hu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Koegel:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Delev:Bristol Myers Squibb: Current Employment. Kostic:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Shah:Bristol Myers Squibb: Current Employment. Wegman:Bristol Myers Squibb: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal